NGeneBio hit the upper price limit on expectations that it would evolve into an AI medical data company by incorporating a solution developed by an LG artificial intelligence (AI) researcher. The solution integrates AI based pathology analysis with the company’s existing next generation sequencing (NGS) capabilities.
QuadMedicine’s share price rose following the announcement that the results of its joint research with global pharmaceutical giant GlaxoSmithKline (GSK) were published in an international academic journal indexed in the Science Citation Index Expanded (SCIE).
In contrast Alteogen saw its share price fall for a second consecutive day, despite announcing a 420 billion won technology transfer deal with a GSK subsidiary.
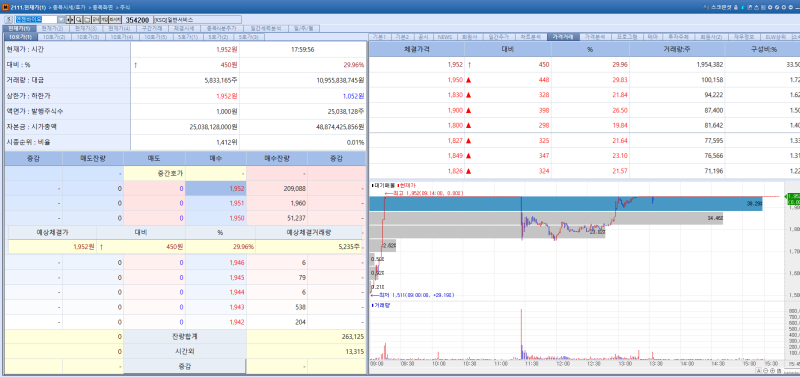
NGeneBio stock trend on Jan 20.(Image=MP Doctor)
◇NGeneBio Aims Beyond NGS Solutions to Become an AI-Powered Medical Data Company
According to KG Zeroin's MP DOCTOR (formerly MarketPoint), NGeneBio's stock surged 29.96% to close at 1952 won on the day. The sharp rise followed the announcement of a licensing agreement with LG AI Research for the "Exaone Path 2.0" solution.
NGeneBio plans to apply the epidermal growth factor receptor (EGFR) mutation detection model to its genomic information management platform "ENGLISH" and sequencing analysis platform "NGAS." The "Exaone Path 2.0" is an advanced precision medicine AI model that can reduce disease diagnosis time from two weeks to under one minute.
The company has secured exclusive licensing rights for the entire Exaone Path 2.0 EGFR solution. Together with LG AI Research, NGeneBio plans to enhance the model to predict microsatellite instability (MSI) and tumor mutational burden (TMB) key markers for determining responses to immunotherapy into specialized solutions named Exaone Path 2.0 MSI and Exaone Path 2.0 TMB, respectively.
NGeneBio has also initiated clinical validation of the Exaone Path 2.0 EGFR model in collaboration with Asan Medical Center. Upon obtaining approval as a digital medical device from the Ministry of Food and Drug Safety, the company intends to gradually integrate the solution into its existing platforms ENGLISH and NGAS.
Ultimately NGeneBio aims to establish a precision diagnostics system by combining clinical genomic data with AI powered pathology image analysis.
A company spokesperson stated, “Exaone Path 2.0 has demonstrated top-tier accuracy in global AI performance evaluations in major pathology diagnosis benchmarks, validating its technological capabilities.
It can rapidly predict the presence of EGFR mutations key biomarkers in non small cell lung cancer by analyzing pathology images, significantly improving diagnostic efficiency and accessibility over traditional tests.”
The spokesperson added “NGeneBio is determined to evolve from a next generation sequencing (NGS) solution provider into an AI driven medical data company that creates new value in healthcare.”
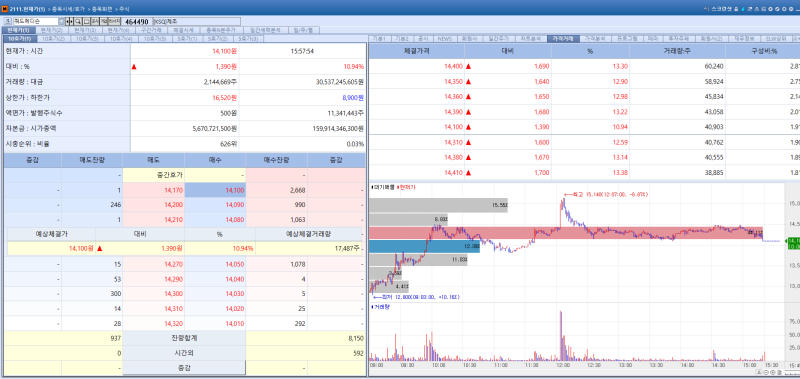
QuadMedicine stock trend on Jan 20.(Image=MP Doctor)
◇QuadMedicine’s Joint Research with GSK on Shigella Vaccine Microneedle Technology Published in International Journal
QuadMedicine’s stock rose 10.94% to 14,100 won, following the announcement that its joint research with GlaxoSmithKline (GSK) on a microneedle based Shigella vaccine was published in an international scientific journal.
The study verified a transdermal delivery method for the Shigella vaccine antigen using microneedle technology. The findings were published in a vaccine focused international journal issued by Elsevier a renowned academic publisher in the science, technology, and medical fields.
Currently, there is no commercially approved Shigella vaccine worldwide. This candidate vaccine has undergone Phase 1 and 2 clinical trials to evaluate its safety and immunogenicity.
In 2022, QuadMedicine and GSK signed a joint research and material transfer agreement to apply QuadMedicine’s microneedle technology to a Shigella vaccine under development by GSK.
Their collaboration has since expanded into research on typhoid vaccines which are already approved yielding promising results.
The published study demonstrated that GMMA (Generalized Modules for Membrane Antigens) based vaccine antigens maintained their quality when applied to two types of microneedle array patches a coated version (C-MAP) and a powder-attached version (P-MAP).
Importantly the study also evaluated the vaccine’s stability under various temperature conditions suggesting the possibility of storage and transport without cold chain requirements.
The publication is considered strong evidence of the practical potential of microneedle based delivery systems as a viable Shigella vaccine strategy.
Following these results QuadMedicine signed an amended agreement with GSK at the end of last month. This new agreement allows the company to begin key non clinical safety studies. QuadMedicine and GSK are collaborating under a milestone based agreement structure.
A company representative stated “This research once again proves the excellence of our proprietary microneedle patch technology. Based on this system we aim to maintain a long term partnership with GSK by providing stable material supply and technical support throughout the clinical and commercialization stages.”
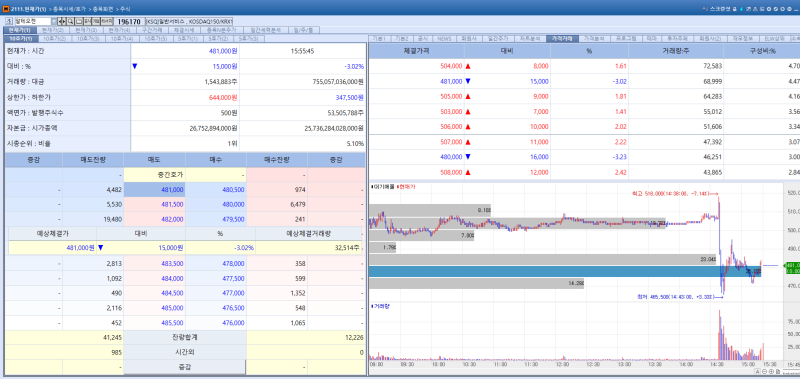
Alteogen stock trend on Jan 20.(Image=MP Doctor)
◇Alteogen Transfers Subcutaneous Injection Technology to GSK Subsidiary
Alteogen’s stock fell 3.02% to 481,000 won, marking its second consecutive day of decline. The drop appears to be due to profit taking following news of a technology transfer agreement with Tesaro a subsidiary of GlaxoSmithKline (GSK). Market disappointment over the deal size contrary to earlier speculation of a multibillion-won contract may also have contributed.
Alteogen signed an exclusive license agreement with Tesaro to develop and commercialize a subcutaneous (SC) formulation of dostarlimab using its proprietary Hybrozyme technology ALT-B4.
Under the agreement Tesaro has secured exclusive rights to develop and commercialize an SC version of GSK’s PD-1 immune checkpoint inhibitor Jemperli by applying Alteogen’s hyaluronidase technology ALT-B4.
Jemperli is GSK’s anticancer drug used to treat advanced recurrent endometrial cancer regardless of biomarker status.
ALT-B4 is a recombinant enzyme protein that breaks down hyaluronic acid a substance that impedes drug penetration in subcutaneous tissues. This creates a pathway in the skin enabling drugs to be delivered subcutaneously instead of intravenously (IV).
Alteogen will receive an upfront payment of $20 million (approximately 29.6 billion won). If key milestones related to development regulatory approvals, and sales are achieved the company may earn up to $265 million (about 392 billion won) in milestone payments.
It will also receive royalties on future product sales. Alteogen will be responsible for supplying ALT-B4 for both clinical trials and commercial production.
An Alteogen spokesperson stated “We are delighted to expand the application of our Hybrozyme technology through this collaboration with Tesaro in the field of oncology. We hope the SC formulation therapy will be successfully developed and launched in the market.”










